Welcome to the ABER Knowledge base
Search content
Webinars, podcasts
and presentations
Applications, posters and journal papers
Frequently Asked Questions
What is capacitance?
In simple terms, capacitance is the ability of a system to store an electrical charge. This principle is used by the Aber capacitance technology to accurately measure biomass. The non-conducting nature of a cell’s plasma membrane allows for the build-up of charge across its membrane when an infinitesimally small electric field is applied by the Aber sensors, and it is this electrical potential that is measured by the technology. Live cells with intact membranes act as capacitors in the presence of this field; the greater the number of live cells in the suspension, the greater the capacitance signal generated and then detected.
What is conductivity?
Conductivity, unlike capacitance, which is the build up of electrical charge, is the measure of the ease with which an electrical charge can flow through a substance. The conductivity is determined by the number of charged ions in the suspension and can be indicative of the production or utilization of ions. Conductivity could possibly provide an estimate of the osmolarity of the suspension.
What is Aber technology measuring?
The Aber sensor provides an automated, real-time, accurate cell concentration and growth profile of the process. Cells with intact plasma membranes can be considered to act as capacitors under the influence of an alternating electric field which is simultaneously created and measured by the Aber sensor. The non-conducting nature of the plasma membrane allows a build-up of charge and this is measured as capacitance in picofarads per centimeter (pF/cm). If required, this can be converted to a relatable unit such as cells/ml, g/L or any other user unit of choice. The capacitance measured is directly proportional to the membrane bound biovolume of viable cells. The Aber sensor also measures the conductivity of the medium, in millisiemens per centimeter (mS/cm). Conductivity is not used to measure biomass but is indicative of the production or utilization of ions by the cell suspension.
Dead cells, solid media particles and gas bubbles are essentially invisible to the technology, as unlike live cells with intact membranes, they are unable to hold charge around themselves.
What are the benefits of using the Aber technology?
The Aber technology can bring benefit to the user’s process by virtue of the following attributes:
- The Aber technology provides real time measurements, giving a complete and detailed profile of the process
- The technology is only sensitive to the presence of live cells, while being insensitive to dead cells, media particles, gas bubbles, etc.
- As the probe is in situ, it reduces or even eliminates the risk of contamination
- With the complete, real time profile of the process, this enables the user to control critical aspects of the process using capacitance technology, such as the feed of complex nutrients, harvest time, cell concentration, and more
- Visualising the profile of the culture in real time also enables troubleshooting, if for instance, the real time profile of the culture deviates from the “golden batch fingerprint”
- To all intents and purposes, the Aber technology has no upper detection limit
- The technology is considered to be plug and play
- Aber capacitance is a mature technology, it has been transitioned from R&D and PD through to cGMP over the past 20 years. Aber has all the necessary certification and validation tools for transition to cGMP
With which biological cells can the technology work?
The technology works with an array of cell types. It is commonly used in mammalian cell culture processes (CHO, HeLa, HEK, Vero, NS0 etc.), microbial, bacterial, yeast, insect cells and fungi; and has more recently been employed in 3D cellular constructs and stem cells applications, specifically with CAR-T cells. Peer reviewed scientific literature is available on the application of Aber technology with various cell types, and can be found here: https://aberinstruments.com/applications/
Can capacitance be correlated with offline counts?
Capacitance can be converted and correlated with any unit of choice familiar to the user via a simple conversion factor. It shows close association with offline counts at all scales.Whilst capacitance can be converted into units such as cells/ml & g/L, there are benefits to using raw capacitance to control processes. This has become increasingly popular, and published most notably by Biogen, who have utilised Aber capacitance sensors to successfully control the feed of complex nutrients. Further information or access to this peer reviewed paper is available through our website: https://aberinstruments.com/application/online-cell-culture-in-cgmp/
Where has the technology been applied successfully?
In addition to being used with a range of cells (please refer to the FAQ “With which biological cells can the technology work?”), the Aber technology has been utilised in, but not limited to, the following applications:
- Monitoring the complete, real-time profile of the process
- Generating a detailed batch fingerprint
- Automatic control of various parameters (e.g feed of complex nutrients, glucose feed, cell concentration etc.)
- Viral Vector and vaccine production
- Identification of infection point
- Harvest point detection
- Microcarriers (Fixed bed and suspension carriers)
- Perfusion
- Seed transfer
- Scale up
- Troubleshooting
- Brewing
- CAR-T
- 3D tissue cultures
The technology has been able to overcome challenging process conditions in an array of applications. The technology is particularly beneficial to microcarrier applications where offline cell counting is innately challenging and error prone, requiring laborious lab procedures. As Aber capacitance technology is only sensitive to the presence of live cells, microcarriers, much like dead cells, gas bubbles and solid media particles are invisible to the technology, enabling the growth profile of the culture to be monitored successfully in situ, in real time.
Relevant publications on these applications can be found on our website (https://aberinstruments.com/applications/) or by getting in contact with our support team.
Is the measurement continuous?
Aber capacitance technology is a continuous, on-line, real-time measurement of the viable biomass of the culture. These measurements are performed every four seconds. This enables you to generate a complete and detailed profile of your process.
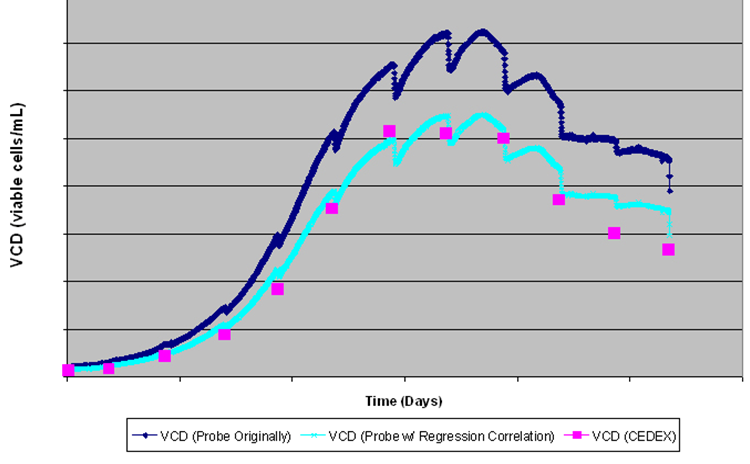
The graph below illustrates the benefits of using an on-line, real time technology such as capacitance relative to an offline method, and demonstrates the value that can be gained by building a complete, detailed growth profile of the culture. On the graph; the dark blue trend represents the raw capacitance in pF/cm, the light blue trend is capacitance which has been correlated with offline counts in cell/ml. Finally, the pink squares represent the offline VCD measured using a CEDEX counter. The characteristic drops in the capacitance profiles represent feed additions, resulting in an artificial decrease in capacitance due to the dilution effect.
However, additional information may be obtained from the real time capacitance measurement. Midway through the process, we observe that the capacitance decreases prior to the feed additions, which indicates that the cells have started dying. Therefore, the user was able to identify the optimum time for feed additions to prolong the culture, based purely on the continuous, real-time capacitance measurement. In contrast, the offline counts provide insufficient detail or knowledge to enable the user to determine the optimum feed time or to recognise cell death in a timely manner to enable intervention, further illustrating the advantages of continuous measurements.
Please note that in addition to capacitance, the Aber sensors also perform continuous and real time conductivity measurements.
Has the technology been used in cGMP?
Aber capacitance technology has been successfully transitioned into manufacturing over the past 20 years and is used by most of the major pharmaceutical companies across the world. All necessary validation tools and certification packages are available to enable cGMP and the technology is compliant with PAT regulatory requirements. For publications describing the use of Aber technology in manufacturing, please contact support@cevans
What is frequency scanning and how can it benefit my process?
In addition to monitoring and controlling critical aspects of a process, the Aber technology can also be used to gain additional insights into the process by means of frequency scanning. Once the technology is established in your processes, frequency scanning could be the next step to further understand and optimise your process. Frequency scanning is achieved by measuring the capacitance over a range of frequencies, from 50kHz to 20MHz. By fitting the scans to a model, key parameters are calculated through the Aber software (SCADA); changes to these parameters may be correlated with critical events in the process. Once the calculated parameters have been correlated with critical events in the process, the parameters may be used to identify process changes in real time, and enable greater process understanding and control, as well as real-time troubleshooting.
One example of how frequency scanning was used to identify a process change was published by Biogen in 2019; where changes in cell physiology were correlated with calculated, derived parameters and used to detect the early stages of apoptosis. The calculated parameters within the Aber SCADA software detected these physiological changes in real time, far earlier than other methods such as trypan blue, enabling timely intervention to reverse apoptosis. For more information, please refer to the publication below:
Ma, F., Zhang, A., Chang, D., Velev, O., Wiltberger, K. and Kshirsagar, R., 2019. Real-time monitoring and control of CHO cell apoptosis by in situ multifrequency scanning dielectric spectroscopy. Process Biochemistry, 80, pp.138-145.